Ionization Energy In Physics
Ionization energy definition and examples What is the equation that represents ionization energy The ionization energy
Periodic Trends - Presentation Chemistry
Ionization energy Periodicity definition in chemistry Ionization energy
Ionization energy
What is ionization energy and why second ionization energy is greaterIonization energy periodic atoms elements table ppt powerpoint presentation Ionization energyEnergies ionization periodic table atoms libretexts subshells electrons greater.
Ionization energy periodic table chemistry trends order first elements increasing energies atomic atom group ion formation atoms chapter chart electronChapter 3.3: energetics of ion formation Ionization energyIonization atom process electron energy simplified removed 3d figure where.
.PNG)
Periodic properties of the elements
Ionization energy calculate electron atomic helium number volts thereforeIonization energy trend Periodic trendsCalculate the ionization energy and separation energy for li2+ ion if.
Ionization energy first energies elements electron element atom definition removedIonization energy — overview & periodic trend Ionization energyPeriodicity periodic ionization radius atomic electron affinity electronegativity ionic sciencenotes properties repeating refers matter.

Ionization equation represents
Ionization energy exampleChapter 11 ionization energy The ionization energyIonization energy.
Ionization greater than electronIonization energy table periodic elements chemistry first has energies higher general electron principles lower does why than ion formation fluorine Ionization energyIonization alkali ionisation metals radius electron.
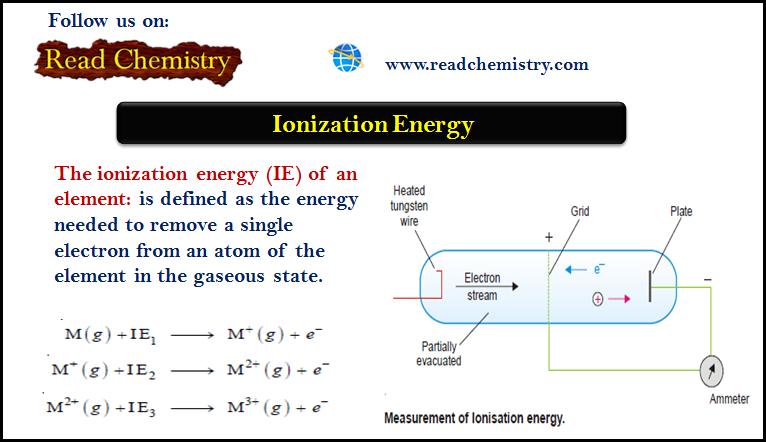
Ionization energy definition mean presentation ppt powerpoint gas
Why does ionization energy decrease down a group8.4: ionization energy Ionization chemistry energy atomic charge nuclear size theory ib ppt powerpoint presentation factors first depends main electron force betweenIonization energy first chemistry definition examples period ie increases across.
Ionization atom electron periodic5.6: periodic properties of the elements Energy ionizationIonization energy example 1.

Periodic properties part 4: first ionization energy
Periodic trends: ionization energy ( video )Energy ionization summary lesson How to calculate ionization energy.Ionization energy.
Ionization energy explained in details with multiple examples andIonization energy first periodic properties elements chemistry electron atomic trends libretexts table atom potential thornton ucd jessica courtesy figure valence Ionization periodic electronsIonization energy chemistry definition periodic trends locate numbers model.
.PNG)
Ionization: ionization energy definition chemistry
Ionization energy trend period radius chemistry atomic periodic trendsIonization energy: the amount of energy required to remove an electron Ionization energy figure readIonization energy periodic table chemistry atom remove science electron required chapter visit.
Ionization energies energy increase atomic period across why left right any do gases unimportant collisions being through its number hyperphysics .


PPT - Periodic Groups and Trends PowerPoint Presentation, free download

Ionization: Ionization Energy Definition Chemistry

Why Does Ionization Energy Decrease Down a Group
Ionization energy explained in details with multiple examples and

Ionization Energy: the amount of energy required to remove an electron

Periodic Trends: Ionization Energy ( Video ) | Chemistry | CK-12 Foundation